Friday, 20 October 2017 Maxim Lyubovsky
Introduction
The amount of solar energy falling to the Earth far exceeds current human consumption. Assuming 300 W/m2 irradiation for 12 hours a day, which is average for the Southwest United States, about 100 quadrillions BTU (~10^20 J) – the amount of energy consumed annually in the U.S. – can be collected from an approximately 100 x 100 mile square. The problem with utilizing renewable energy, however, is its highly dispersed nature. Solar and wind energy is distributed over large areas at a relatively low density. With the above irradiation assumptions, collecting 30 kW power to move a small car would require an area of 10×10 meters. Unlike oil and gas where only a small wellhead installation on the surface enables extraction of large amounts of energy, wind and solar necessarily have to be collected over large swaths of land. To be economically viable this should be low utilization land such as deserts or mountains, generally far removed from populated areas where the energy is ultimately consumed. The clean energy challenge, therefore, is not in finding energy – wind and solar alone far exceed human needs – the challenge is in converting the energy into a form suitable for consumption, accumulating it and delivering it to the consumer on demand and at competitive cost.
Present day energy carriers
Figure 1 shows the energy flow diagram for the United States. Currently, solar and wind energy are utilized almost exclusively as electricity, and other than via electrified transportation do not make inroads into the transportation fuel market. Yet electricity accounts for only about 18% of energy delivered to consumers (12.71 out of total of 72.86 quads consumed by all sectors combined). While large investments directed in increasing the use of electricity in the industrial and transportation sectors have been made, complete replacement of natural gas and petroleum carriers by electricity (even if technologically feasible) would require nearly 5-fold increase in the electrical grid capacity, an extremely large and costly undertaking.
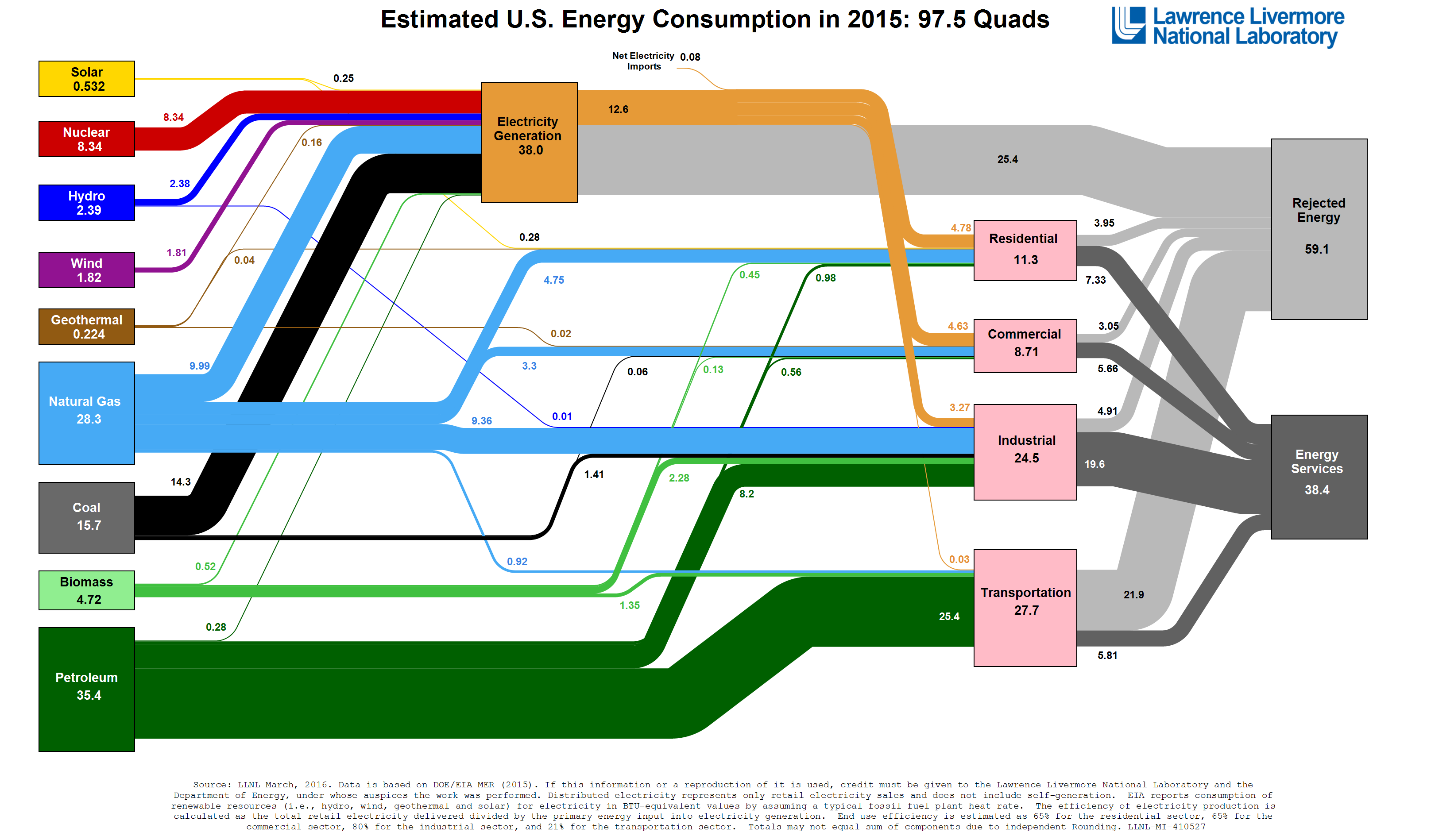
Figure 1. 2015 U.S. energy flowchart.
Figures 2 and 3 show distribution of solar and wind resources in the United States. Renewable resources are concentrated in the Southwest and Great Planes regions, but population is highest on the coasts and there energy is ultimately consumed. Figure 4 maps locations of electric generating facilities throughout the U.S. It shows that today electricity is generated in close proximity to consumers to avoid long distance transmission of electricity on a large scale. Coal and natural gas, for the most part, are used to bring energy to the population centers where it is converted to electricity.
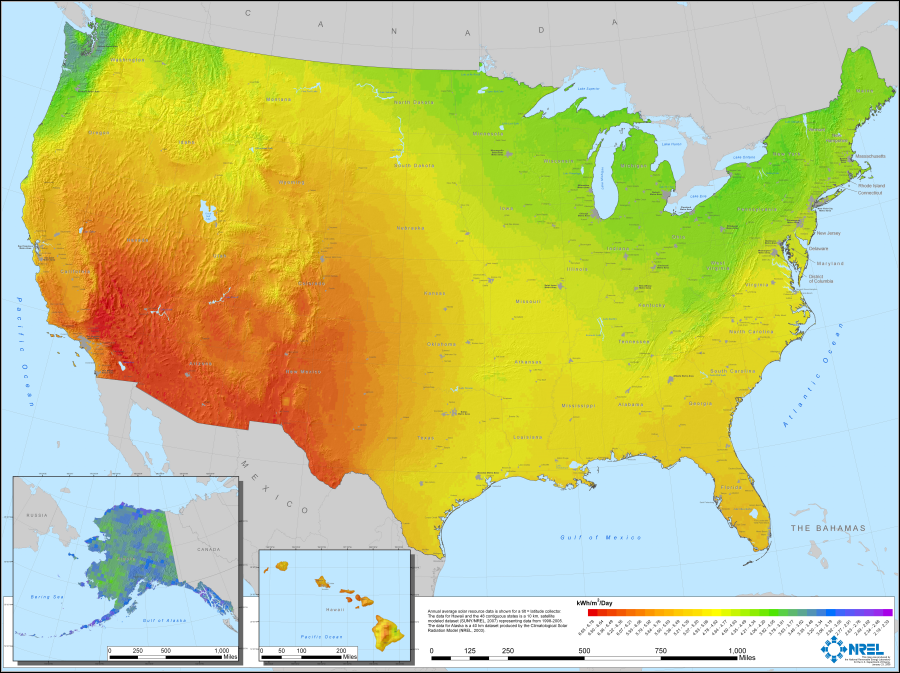
Figure 2. U.S. Photovoltaic Solar Resource Map

Figure 3. U.S. Wind Resource Map
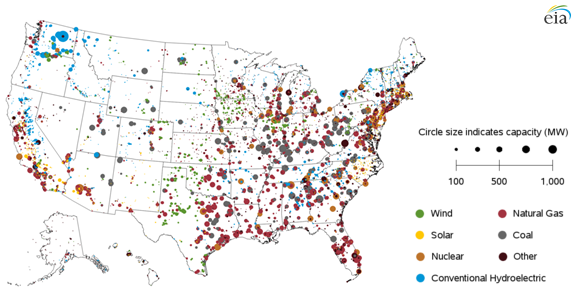
Figure 4. Operable Utility-scale generating units as of Sept. 2015.
Transmission of energy through electric wires is much more expensive than transportation of fossil fuels. The direct cost of building a two circuit 500 kV 3-phase AC transmission line with peak capacity of 3000 MW through a rural area is estimated at about $5 million per mile. For transmission distance of about 1000 miles the cost of transmission is estimated at about $76/MWh. Furthermore average transmission losses in power lines are about 6% and at peak power (peak current loads) can increase to as high as 20%. For oil transportation in railroad cars the average cost is about $10-15 per barrel for distance of about 1000 miles. This translates to about $6.3-9.4/MWh. Oil transportation through pipelines is even less expensive. While the cost of both electricity and oil transportation may vary widely depending on local conditions, on average energy transportation over long distances in the form of liquid fuel is about an order of magnitude less expensive than in the form of electric current.
To compete with established fuels renewable energy carriers must have comparable energy density and cost. A comparison of several common liquid and gaseous chemicals that are used or often considered as energy carrier options is shown in Table 1. The energy density was calculated by normalizing the lower heating value (LHV) of a chemical to the volumetric density under specified conditions (propane and ammonia are shown as liquids under saturation pressure, which is less than 20 bar for temperatures <50oC). The price range was estimated by normalizing wholesale commodity market prices to the LHV of the fuel. For hydrogen the commodity market is not as established as for other chemicals, so the US Department of Energy (DOE) target for hydrogen fuel at $4/kg (~10/MSCF) was used for the lower range and $20/MSCF (~$8/kg), which is the approximate contract price for cryogenic H2 delivery was used for the higher range.
Not surprisingly, diesel and gasoline have the highest power density. This was one of the important factors that helped internal combustion powered cars win domination over alternative technologies in the early 20th century. Natural gas is by far the least expensive fuel option, but it has much lower energy density even at high pressure. Low price makes natural gas very attractive for electricity generation and industrial use, where it can be delivered through established pipelines, but low power density hampers its broad scale use as vehicular fuel.
Table 1. Common fuels and chemicals power density and price range.
| Fuel | Conditions | Energy density kWh/liter | Market price range | Units | Normalized price range $/MWh |
| Diesel | ambient | 9.9 | 1.5 – 3 | $/gal | 40 – 80 |
| Gasoline | ambient | 9.7 | 1.5 – 3 | $/gal | 40 – 80 |
| Ethanol | ambient | 5.9 | 1.5 – 3 | $/gal | 70 – 140 |
| Methanol | ambient | 4.4 | 1 – 2 | $/gal | 60 – 120 |
| Propane / LPG | ~ 20 bar | 6.6 | 1 – 2 | $/gal | 40 – 80 |
| Ammonia | ~ 20 bar | 3.5 | 300 – 600 | $/MT | 60 – 120 |
| Natural gas | 250 bar gas | 2.7 | 3 – 6 | $/MSCF | 10 – 20 |
| H2 (gas) | 700 bar gas | 1.3 | 4 – 8 | $/kg | 120 -240 |
| H2 (cryogenic) | -253 oC | 2.4 | 10 – 20 | $/MSCF | 125 – 250 |
Ethanol and methanol can be made from renewable energy sources and are liquid under a normal range of ambient conditions. Their energy density is respectively about two thirds and half of that of petroleum fuels. Ethanol is produced in large quantities in bio-refineries from grains, and more recently also produced from cellulosic biomass, and is used as additive to gasoline in E10, E15 and E85 fuels.
Over the last decades hydrogen has attracted much attention as a possible clean energy carrier, particularly in the transportation sector. Hydrogen fuel would likely be produced locally from natural gas, grid electricity or from renewable fuels in a same way as electricity is generated in close proximity to the consumers, as low energy density of hydrogen even at very high pressure makes it an unlikely choice for a large-scale energy carrier over long distances.
Renewable liquid fuel option
While the physical properties of hydrogen gas make it a poor choice of energy carrier, chemical properties of hydrogen make it a unique energy intermediate. Electrical or solar energy can be directly and efficiently converted into chemical energy by splitting water into hydrogen and oxygen, storing about 40 kWh of energy per kilogram of separated hydrogen. Water electrolysis is a well-established technology with commercial multi-MW scale units producing hydrogen at a rate of tons per day. A range of other technologies for advanced water splitting directly by sunlight are being investigated. With more efficient and lower cost electrolysis technologies, electrolyzer units may be directly coupled with remote wind turbines and solar panels to produce hydrogen from these renewable energy sources. The high chemical activity of hydrogen allows it to easily react with other substances to create liquid fuels. In fact, the vast majority of hydrogen produced today is utilized in oil refineries, ammonia production and methanol synthesis. The same chemical reactions can be used to convert hydrogen produced from renewable energy into liquid energy carriers that can be easily stored, transported and distributed to energy consumers.
Ammonia, which is liquid under pressures above ~20 bar, can be produced by combining hydrogen with nitrogen in a Haber–Bosch process, which has been in the core of fertilizers and chemicals production for more than a century. The nitrogen required for the process is separated from air, a well-established energy intensive industrial process. The high strength of the N-N bond requires high temperatures to activate the reaction, which in turn requires the process to operate at high pressure to overcome thermodynamic constrains. Haber-Bosch reactors generally operate at temperatures about 450-500oC and pressures up to 300 bar. One disadvantage of ammonia as an energy carrier, outside the need for elevated pressure to keep it in liquid form, is that its use as fuel would require building essentially new infrastructure. Still, distributed production of ammonia utilizing renewable hydrogen to replace ammonia produced from natural gas in fertilizer production, particularly for local agricultural use, may be very attractive.
Fischer-Tropsch (F-T) synthesis is another industrial process utilizing hydrogen to produce liquid hydrocarbon fuels directly compatible with the existing gasoline and diesel infrastructure. Currently F-T plants utilize natural gas which is reformed into a mixture of CO and H2 (syngas) and then to synthetic hydrocarbons, hence this technology is often referred to as Gas-to-Liquid (GTL). Several large GTL projects with tens of thousands of barrels per day capacity have been deployed around the world. F-T reactors generally operate at lower temperature and pressure than that required in ammonia synthesis at T~230-240oC and P~25-40 bar. In order to utilize renewable hydrogen instead of NG in F-T synthesis, the CO required for the process can be produced from captured CO2 (discussed below) in a Reverse Water-Gas-Shift reaction, which is also a well-developed industrial process.
Methanol can be produced by directly reacting hydrogen and CO2. Methanol production is also an established industrial process with several large scale plants producing methanol from natural gas or coal at million tons per year capacity. Methanol synthesis reactors operate at P~40-100 bar and T~220-280oC. Methanol is also commercially produced by variety of small plants having capacity as low as several tons per day. These smaller plants can be adopted to utilize renewable hydrogen produced by 10-20 MW electrolyzers, and be directly integrated with wind or solar farms.
Methanol is liquid under ambient conditions and can be blended with gasoline in the same way as ethanol. With minor engine modifications methanol and its derivatives can be used directly as internal combustion fuel. It also can be converted into gasoline or other common fuel grades through the MTG process demonstrated by ExxonMobil. Alternatively, it can be easily converted back to hydrogen at the point of use. Methanol is the simplest alcohol molecule and is bio-degradable by bacteria naturally present in soil and ground water. This is an attractive property for a fuel as any spills would be naturally disappearing within about two weeks.
Converting renewable hydrogen into liquid hydrocarbons through Fischer-Tropsch or methanol synthesis requires adding CO2 to the process. This CO2 can be captured from stacks of power plants and industrial furnaces. Several large carbon capture projects with capacity up to 1 million tons CO2 per year have been put in operation in the USA in recent years. In these projects CO2 is separated to high purity, compressed to supercritical fluid pressure of about 100 bar, transported over hundreds of miles by pipelines, and injected deep underground for enhanced oil recovery (EOR) in oil fields or for permanent storage in geological formations.
Technologies for transporting CO2 are also well developed. A network of CO2 pipelines exists to move CO2 from capture sites to oil fields where it is used for EOR. On a smaller scale CO2 is transported by tanker trailers as refrigerated liquid at about -30oC and 5-10 bar pressure (CO2 converts to solid “dry ice” if cooled at ambient pressure). Eventually the demand for CO2 for renewable fuel production would likely lead to development of Direct Air Capture (DAC) technologies collecting CO2 from ambient air, which will eliminate the need for CO2 transportation. DAC systems would be co-located and integrated with the rest of the renewable fuel system. It is often argued that DAC would not be feasible because very low concentration of CO2 in the ambient air would require excessive energy for separation. The thermodynamic minimum work required for CO2 separation from air at ambient temperature of 300K and CO2 concentration at 400 ppm is about 19.5 kJ/mole_CO2. When CO2 is utilized in synthesis of liquid fuel this separation energy should be compared with the energy required for the production of hydrogen. Thermodynamic energy for splitting water is 285.6 kJ/mole_H2 and three moles of H2 are needed per mole of CO2, so that production of hydrogen requires about 40 times more energy than separation of CO2 from air. Developing DAC processes therefore, has engineering rather than thermodynamic restrictions, which can be successfully overcome if there is sufficient market drive.
Recent review of DAC suggests that cost of these technologies is currently too high and would need to be significantly reduced before practical applications are possible. Still several companies are developing DAC technologies in an effort to bring the cost down. Integrating DAC with liquid fuel synthesis would allow positioning the renewable fuel systems in remote locations where land is inexpensive and steady and consistent wind or solar power is available, irrespective to proximity of CO2 sources.
Cost estimate for Wind-to-Fuel production
An estimate for the cost of methanol production from renewable hydrogen and captured CO2 based on the results of independent studies on the cost of renewable electricity, water electrolysis, CO2 capture and methanol production from natural gas is shown in Table 2.
Table 2. Cost estimates for methanol production from H2 and CO2.
| Cost of H2 production by PEM electrolysis at 1500 kg/day scale | $4.23 /kg_H2 |
| Electricity component in electrolysis H2 cost @ $0.0688 /kWh | $3.46 /kg_H2 |
| Levelized PPA for onshore wind power | $0.0235 /kWh |
| Cost of H2 production by PEM electrolysis @ $0.0235 /kWh | $1.95 /kg_H2 |
| H2 in MeOH (kg H2 per kg MeOH) | 0.19 kg/kg |
| Cost of H2 in MeOH | $1.10 /gal MeOH |
| Assumed cost of CO2 capture | $40 /tonne_CO2 |
| CO2 in MeOH (kg CO2 per kg MeOH) | 1.38 kg/kg |
| Cost of CO2 in MeOH | $0.17 /gal MeOH |
| Capital and O&M cost in MeOH synthesis | $0.5 /gal MeOH |
| Cost of MeOH produced from H2 and CO2 | $1.77 /gal |
The cost of renewable hydrogen production by an electrolyzer coupled with a wind turbine is based on the cost analysis of a 1500 kg/day PEM water electrolysis system.1 For renewable hydrogen production the electricity cost component was reduced proportionally from the grid electricity price of $0.0688/kWh assumed in 1 to the estimated wind electricity PPA cost of $0.0235 /kWh.2 This resulted in hydrogen cost of $1.95/kg, which is consistent with current DOE estimate for the cost of hydrogen production. Note, that when an electrolyzer is integrated directly with a wind turbine, the electricity cost may be even lower than the PPA assumed in 2 as the costs for power conditioning and transmission would be excluded. Also when an electrolyzer is co-located and integrated with a methanol synthesis plant the costs for hydrogen compression and transportation will be eliminated, as the pressure required for methanol synthesis will be produced directly by the electrolyzer cell.
For the cost of CO2 the DOE carbon capture cost target of $40 per metric ton CO2 was assumed in this estimate, as the exact cost of CO2 capture and storage in the existing large-scale CCS projects remains commercial confidential information.
The amount and cost of H2 and CO2 in methanol production is calculated based on stoichiometry of methanol synthesis. This assumes 100% conversion of H2 and CO2 into methanol. While this is a somewhat optimistic assumption, nearly complete conversion can be achieved by recycling unconverted reactants after removing product methanol and water, which is a common design for methanol synthesis plants. Methanol synthesis is an exothermic process with about 1.55 MJ of heat released per kilogram of produced methanol. This heat is usually removed into boiling water and can be utilized in other parts of the plant, e.g. in a separation column, so that less or no extra energy is required. Any additional power for the operation of the balance of plant components would be supplied by the primary wind or solar source.
Capital and O&M costs for methanol synthesis was adopted from 2014 NETL study of the cost of methanol production from coal and natural gas (NOTE: the NETL numbers include the cost of CO2 capture).3 Note that this O&M costs assumption is likely to be an overestimate as the expensive high-temperature steam methane reforming or coal gasification section of the methanol plant will be eliminated when starting the process with H2 and CO2 feed instead of natural gas or coal.
With this set of assumptions the cost of methanol produced from renewable H2 and captured CO2 is estimated at about $1.8 per gallon (~$590/MT or ~$106/MWh). For comparison the NETL study estimates the cost of methanol production from natural gas including CO2 capture at ~$0.8 per gallon at natural gas price of $3/MMBtu and the cost of methanol production from coal including CO2 capture at ~$1.6 per gallon at coal price of $2/MMBtu. The estimated cost for renewable methanol production falls into the range of historic wholesale methanol market price variation shown in Figure 5.
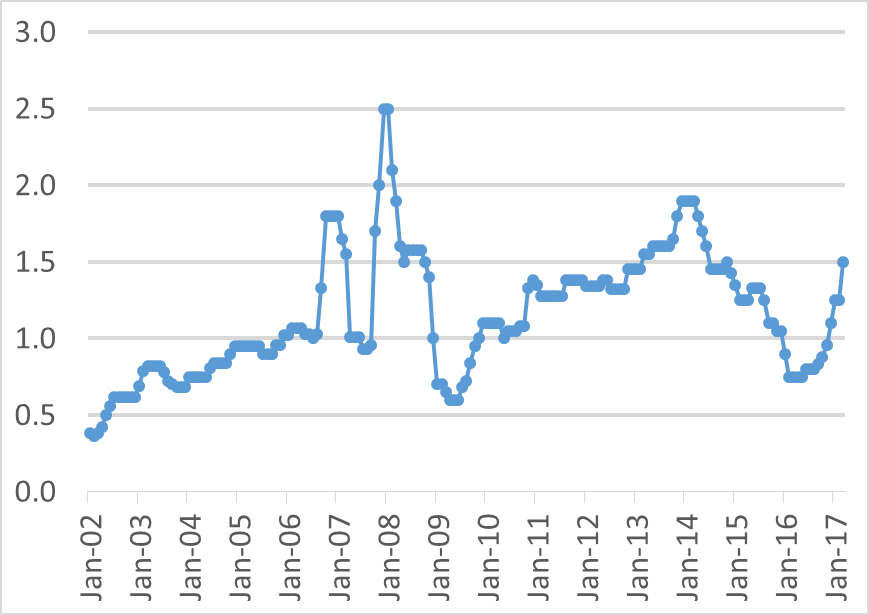
Figure 5. Historic wholesale market price for methanol, $/gal. Source: Methanex.
Two recent papers report on technoeconomic analysis of methanol production from captured CO2 and renewable H2. A.Tremel et.al estimated the cost of methanol production at €0.175 /kWh (~€2.9 /gal).4 The analysis compares several renewable fuel options and mainly based on applying scaling factors between the existing large-scale processes and renewable systems and does not consider modifications of the processes necessary for integration with renewable energy supply. M.Pérez-Fortes et.al conducted modeling study of a renewable methanol plant operating with captured CO2 and hydrogen produced by water electrolysis.5 They estimate breakeven price of methanol at €723.6 /MT (~€2.2 /gal). Their model, though, considers CO2 capture and H2 production to be outside the system boundary and does not account for their integration. For example, their model provides for a multi-stage compression of H2 feed, which is estimated to constitute 45% of the total plant cost, while high-pressure hydrogen can be produced directly by an advanced water electrolysis unit. For an accurate cost analysis it is critically important to consider integration of the whole system and consider possible cost reducing alterations, such as avoiding DC/AC and AC/DC conversion, hydrogen drying and compression, utilizing heat released in methanol synthesis, etc.
The cost estimate suggests that the cost of renewable methanol needs to be further reduced to be competitive with the methanol produced from natural gas, especially at current low natural gas prices in the US. Yet even at the existing level of technology development the renewable methanol may be competitive with the coal based methanol, which constitutes a large fraction of global methanol production, particularly in China. Increasing use of methanol produced from natural gas as vehicular fuel and in other energy applications in the near term may become a gateway for gradual conversion to renewable methanol as the cost of production decreases.
While the cost analysis provided here is only a rough estimate, it indicates the critical issues that need to be addressed to reduce the system cost and produce cost competitive renewable fuels. The cost analysis indicates that renewable hydrogen constitutes more than 60% of the cost of renewable methanol, while the cost of CO2 makes a relatively small fraction of it. Lowering the cost of renewable hydrogen therefore, is the key to producing cost competitive renewable synthetic liquid fuels. This favors placing the fuel production systems in locations where abundant and reliable wind or solar resources are available, land is inexpensive and the cost of renewable energy is low, while the cost of transporting CO2 to these remote sites should not significantly increase the overall cost of fuel production. With the low cost of the primary renewable energy sources (essentially the cost of land under the renewable fuel system installation) the system components, individual technologies, design tradeoffs, and operating parameters should be selected to minimize the system capital cost even at possible expense of lower efficiency.
Table 3 shows an estimate for methanol production rate for a renewable fuel system integrated with a 10 MW power source. This amount of power, which can be produced by several wind turbines or a solar farm was selected to approximately match the size of the electrolyzer analysis in 3 and the production size of small methanol plants. The analysis assumes 100% system utilization and results in process efficiency estimate of 57% (assuming LHV of produced methanol). Detailed design of the system integration and assessment of power loads of the balance of plant components are required for a more accurate estimate of the process output and efficiency.
Table 3. Estimate of methanol production rate.
| Wind or solar power supply assumption | 10 MW |
| H2 production assuming @ 54.6 kWh/kg_H2 | 4400 kg H2/day |
| CO2 demand | 32 metric ton/day |
| Methanol production | 23 metric ton/day |
| 186 barrel/ day |
At least two pilot plants producing renewable methanol have already been demonstrated. Since 2012 Carbon Recycling International is operating a George Olah 4000 metric ton per year renewable methanol plant at Svartsengi, Iceland producing methanol from hydrogen generated from water electrolysis utilizing Iceland’s geothermal and hydro power and from CO2 captured from geothermal power plants. In Japan Mitsui Chemicals has demonstrated operation of a 100 metric ton per year renewable methanol pilot plant. The plant was utilizing CO2 captured from local industrial emitters and hydrogen produced by photocatalytic splitting of water.
Discussion
Converting wind and solar energy into common liquid fuels at costs competitive with the fuels produced from oil or natural gas will open ways for renewable energy penetration into the existing fuels infrastructure. Renewable energy in the form of liquid fuels can be utilized in all sectors of the economy currently served by petroleum products and not be limited to electricity grid applications. Development of the renewable liquid fuels technologies would remove several barriers currently impeding expansion of renewable energy use.
Converting wind and solar electricity into easily transportable liquid fuels would divorce wind and solar projects from the electricity grid and enable their spread into remote, scarcely populated areas having ample and reliable wind and solar resources which are currently not accessible because of the high cost of electricity transmission. It will also provide a nearly infinite storage capacity for renewable energy and thus help avoid the “curtailment” problem that restrains intermittent renewable energy penetration into the electrical grid.
Implementing technologies for converting renewable energy into hydrocarbon fuels would create demand for CO2 and thus establish a market for CO2 capture. Initially CO2 is likely to be captured from concentrated point emission sources, such as coal fired power plants, cement plants, etc. Developing the market for CO2 will lead to lowering the costs for CO2 capture and to propagating the technology to more dispersed and difficult to capture sources. Eventually the cost of direct air capture may be sufficiently reduced so that DAC can be integrated with renewable fuel production on large scale. This will provide additional freedom to install the fuel production plants in remote locations. Combining DAC with liquid fuel synthesis from wind and solar energy will essentially create an artificial “photosynthesis” process and carbon cycle akin to the artificial nitrogen fixation process developed by chemical engineers in the beginning of the 20th century.
The cost analysis in this paper suggests that renewable hydrogen constitutes the most significant fraction in the renewable liquid fuel production cost. Reducing costs of advanced water splitting technologies, therefore, is the key to renewable energy utilization. While water electrolysis is a well-established commercial technology its broad adoption is currently hindered by the high cost of grid electricity and by competition from hydrogen produced from natural gas in industrial scale steam reforming plants. There is room for technology optimization and dramatic cost reduction from the current levels, and this would likely be sparked by the increased demand brought about by expanded renewable liquid fuel production. Development and deployment of low-temperature and high-temperature electrolysis as well as alternative technologies for direct hydrogen production by sunlight such as photoelectrochemical (PEC) and solar thermochemical (STCH) water splitting which provide potential to dramatically reduce the cost of renewable hydrogen are being pursued as part of H2@Scale DOE initiative.
Synthetic liquid fuel production systems will be utilizing highly dispersed wind or solar primary energy sources. This would necessitate that each individual project be relatively small, comparable in size and cost with a wind turbine or a utility solar farm installation. Economy of scale would be achieved by replicating identical plants in multiple locations over vast swaths of scarcely populated areas. As the feeds to the process, namely wind, sunlight, water and CO2 will be the same at every location no modifications to the systems would be required. Because of the relatively small size of each system the financial risk of developing the initial demonstration projects would not be very high.
Conclusions
There is economic opportunity in funneling solar and wind energy into the transportation fuel market by utilizing these resources to make synthetic liquid energy carriers compatible with present infrastructure. Methanol may be an attractive choice for such an energy carrier as it can be readily produced from hydrogen obtained with renewable wind and solar energy and from captured CO2 and be utilized in a wide range of existing and developing energy applications. The cost of renewable hydrogen constitutes about 60% of the total cost of renewable methanol production, therefore, lowering the cost of water splitting technologies is the key to developing low cost synthetic renewable liquid fuels.
Maxim Lyubovsky, ORISE Fellow at Fuel Cell Technologies Office, US Department of Energy. He can be reached at maxim.lyubovsky@ee.doe.gov
Acknowledgements.
This research was supported in part by an award from the Department of Energy (DOE) Office of Energy Efficiency and Renewable Energy Science and Technology Policy Fellowships administered by the Oak Ridge Institute for Science and Education (ORISE) for the DOE. ORISE is managed by ORAU under DOE contract number DE-AC05-06OR23100. All opinions expressed in this paper are the author’s and do not necessarily reflect the policies and views of DOE, ORAU, or ORISE.

